global data intelligence: nyquistmedtech
Make Confident, Data Driven Decisions
NyquistMedTech provides comprehensive global clinical, regulatory, and medical device data across key markets, from large markets like the US to emerging markets like China. Our platform incorporates clinical trials contributing to approval success, FDA approval documents, product development roadmap, device genealogy revolution, Posticates for identifying innovators, and insights from adverse event data. With NyquistMed, you’ll gain access to mission-critical insights to accelerate your time-to-market.
Clinical Trial Data
- Get 20% more than the golden standard of clinicaltrial.gov by discovering trials that are outside the US in Japan, China, and more
- Instantly uncover deeply-buried intelligence and break down the languages barriers by providing English translation for OUS
- Access an AI-generated competitive landscape to help you save time finding the needle in the haystack
Business Development
- Enrich your global strategy by differentiated and detailed insights from the largest emerging markets such as China
- Utilize AI-powered similar devices to help you identify unexplored sectors and indications
- Set alerts to track changes in the pipeline, product, adverse events, and recalls so you never miss a catalyst event
Regulatory Approval Planning
- Gain in-depth comprehensive insights across the world to make an informed regulatory strategy
- Infuse fun into MAUDE with AI, which will summarize AE problems and aid in risk identification for your product
- Utilize AI to make sense of all inspections and recalls, presenting you with visually engaging data to tell the story effectively
All the Data, Insights, and Tools Needed to
Accelerate Your Innovation
Unparalleled Global Data
global clinical trials
Decode the Global Pipeline for Breakthrough Success
Save time and effort in global trial site research, collection, cleaning, and analysis with our Clinical Trial module. Unlike traditional databases, our AI comprehends context and connects information seamlessly. Forget multiple filters and use natural language search to find trials by indication, technology, patient type, and more.
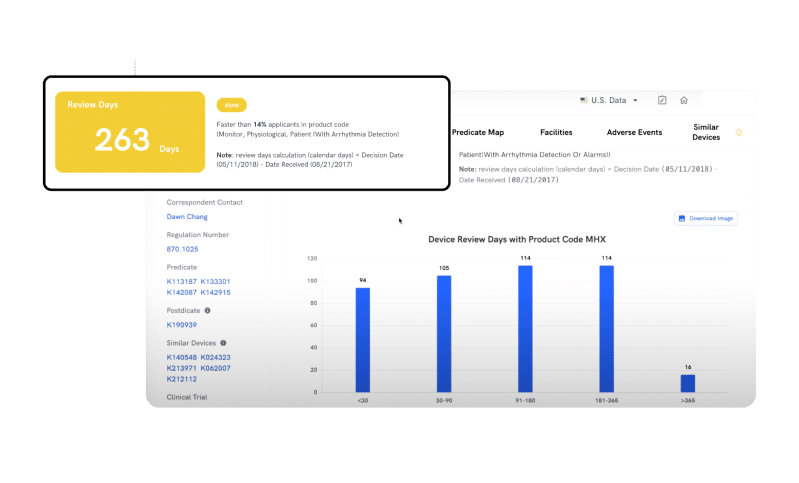
REVIEW TIME
Plan Your Path to Approval and Save Precious Time
Remove uncertainty and effortlessly estimate clearance timelines for your devices. By leveraging the Review Time feature, you can now benchmark against peers and track timelines for similar devices in your product code, improving your regulatory planning process and saving precious time.
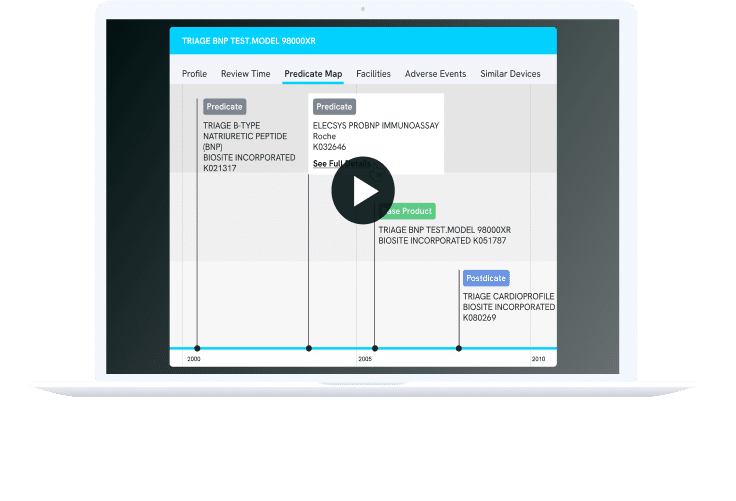
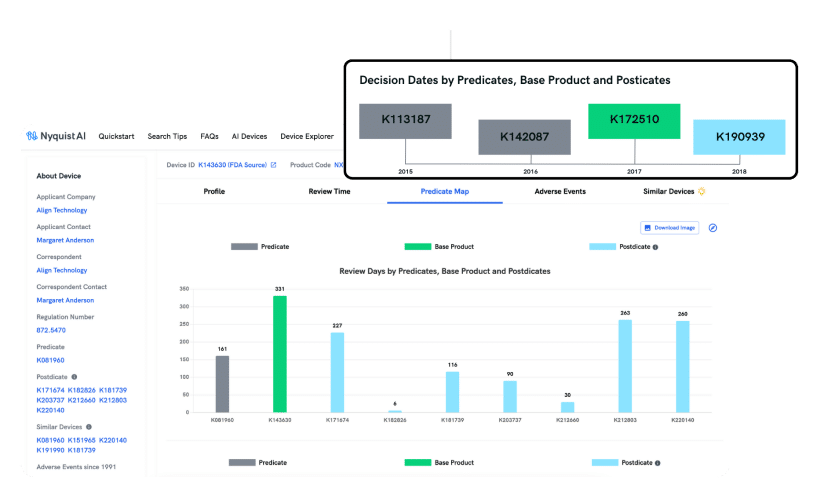
PREDICATE MAP
Improve Your Probability of Regulatory Success
Discovering the ideal predicate is paramount for a successful 510(k) clearance. NyquistMed empowers you to uncover predicates effortlessly, whether based on indication, technology, or natural language descriptions, mirroring how your engineering team conveys the product to regulatory authorities.
Experience the Future of Innovation
with Global Intelligence Platform: NyquistMed
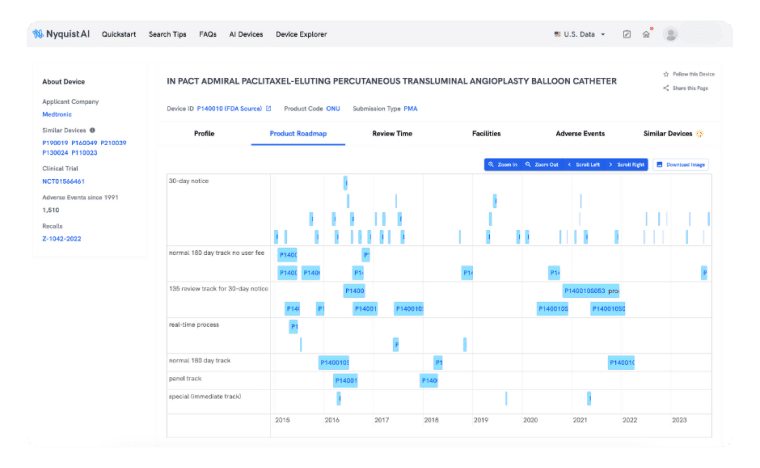
product roadmap
Plan Product Development with Confidence
Preparing a PMA can be slow and cumbersome, but not with Product Roadmap. We simplify the collection, analysis, and extraction of precise insights throughout the entire product launch cycle. Gain a comprehensive view of how prior successes expand indications, modify labels, and improve design seamlessly.
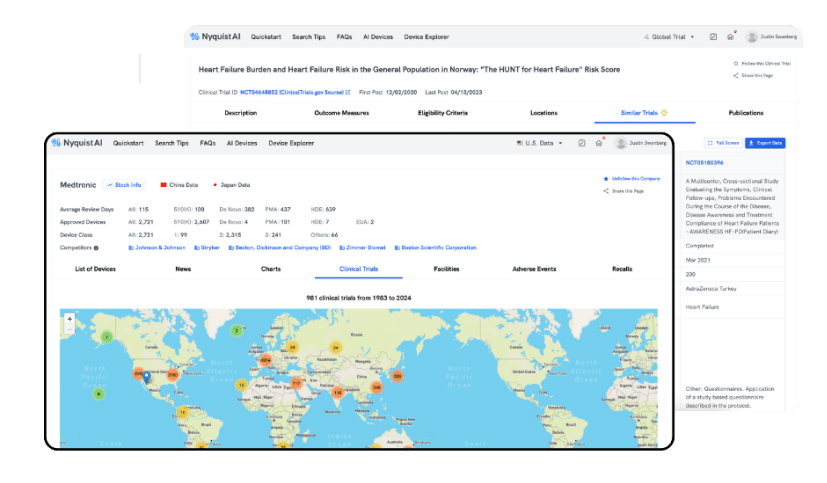
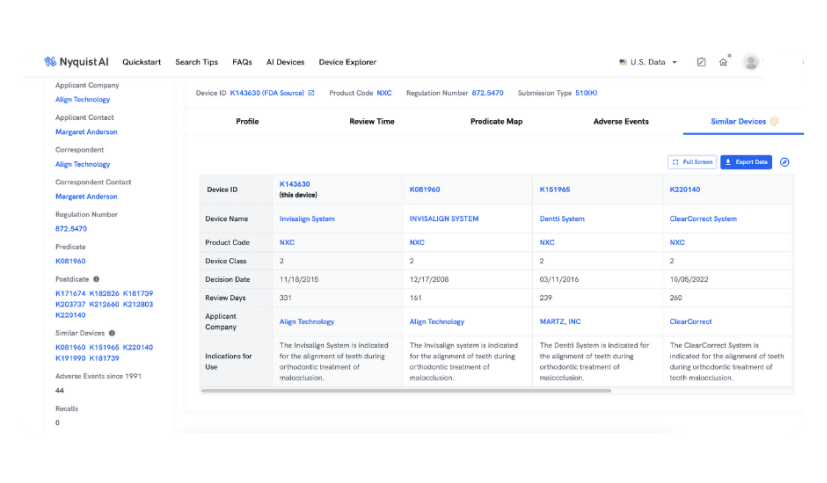
similar devices
Automate Competitor Landscape Research
Our AI excels at contextual understanding and connecting the dots. Utilize Similar Devices to assess the competitive landscape and effortlessly discover devices from various sectors that address the same indications. With just one click, our AI instantly presents the top five similar devices based on your query.
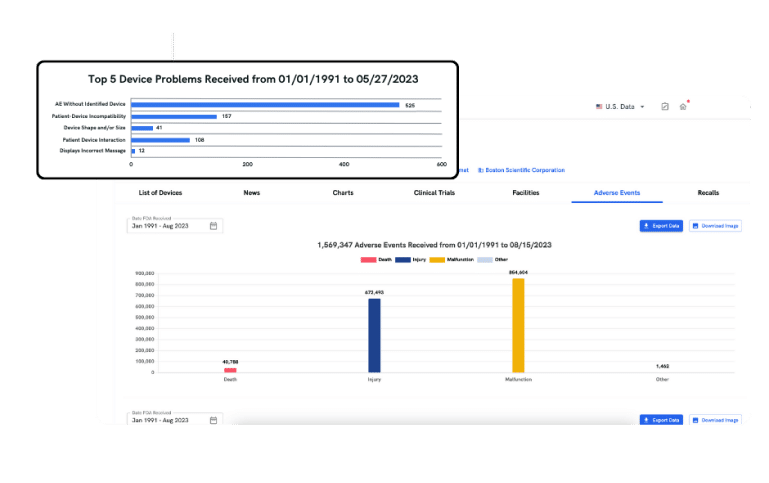
adverse events
Discover Important Risk Signals
Browsing the MAUDE adverse event database can be time-consuming and manual. With adverse events, you gain quick access to essential safety data and critical insights, helping you detect significant risk signals, benchmark against peers, and identify trends from vast data.
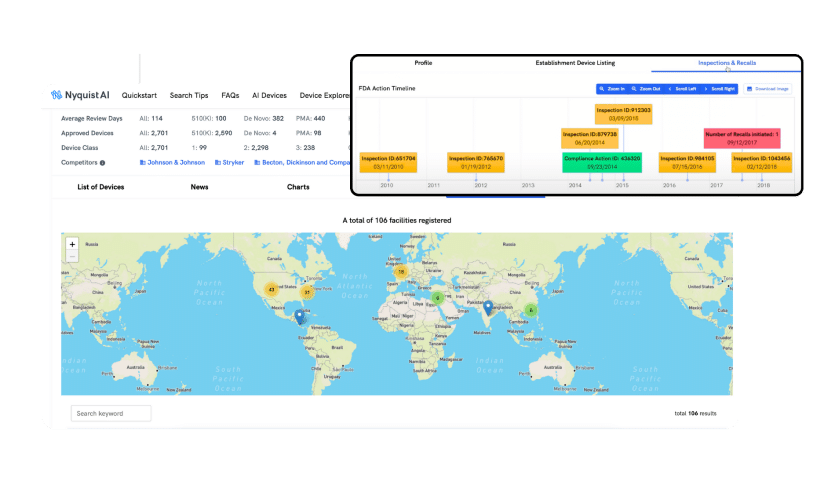
FACILITIES
Discover and Monitor All Possible OEM Facilities
Quickly explore the registered OEM facilities available worldwide. Determine which is best for your device by reviewing product manufacturing history, inspections, citations, or recalls. You can also track your own facilities' inspections and benchmark them against your competitors.
Why Our Clients Love NyquistAI
“I use the NyquistAI platform several times a week. I previously would search several different databases, use CTRL-F to find information, and verify again through Google. I could have easily missed something since it was harder to search using my previous methods. I feel that we get a more accurate result from this platform and I am more confident with the search results from NyquistData.”
Sam Eakes
Vice President, Regulatory Affairs Greenleaf Health
“My work is unique in that I work with a team of engineers on projects at earlier stages of innovation. My role is to provide regulatory knowledge and input in the early planning phase. I use the NyquistAI platform quite frequently and I find it to be a very user-friendly tool for potentially identifying novel pathways for the regulatory process. This tool has helped me save a tremendous amount of time and headache in my work.”
Krystal Santiago
Director RA/QA/CA NXT Biomedical
“I’m interested in improving our workflow and getting information that I need as quickly as possible. I’m impressed with the results generated from your product. When my team members tell me that they can find the information they need in 30 minutes instead of several hours, that saves us time and money.”
Dan Schultz
Principal, Medical devices & combination products Greenleaf Health
“For my area of work in strategy development and innovation, we have a very limited set of database resources. Typically I have to do a lot of Google searches to find some information and spend significant time filtering the information to find useful insights. NyquistAI really solved one of our urgent business problems by creating transparency and agility on some key information. I am so glad that I have the resources of NyquistData for my work.”
Kelly Lu
Ph.D., Program Director, Cardiac Rhythm Management Medtronic
Frequently Asked Questions
In the rapidly evolving landscape of life sciences, staying ahead means harnessing the power of data. This is where NyquistAI stands out as the quintessential partner for your company. As the sole AI platform specializing in global clinical and regulatory data, NyquistAI offers unparalleled insights and intelligence.
Strategic Advantage:
- Speed: NyquistAI transforms the way your team accesses information. Decades of data are synthesized in seconds, unveiling insights that were once invisible.
- Innovation: Originating from Silicon Valley’s elite Google Accelerator, NyquistAI is at the forefront of technological advancement.
- Global Reach: As a preferred vendor for industry giants like Astellas, Medtronic, and BD, NyquistAI’s reputation is global and trusted.
Operational Excellence:
- Expertise: By combining subject matter expertise with augmented intelligence, NyquistAI delivers not just data but practical, actionable solutions.
- Efficiency: Users report that an hour in NyquistAI equals 6-8 hours of traditional research. This efficiency translates to significant cost savings.
- ROI: With moderate use, NyquistAI’s ROI calculator estimates an annual return of approximately $70-80k per user in time saved.
Your Next Step: Leverage this RFP template to articulate the benefits to your supervisor. With NyquistAI, opportunities that were previously out of reach are now within your grasp. Accelerate your approval process and join the ranks of top innovators who have already embraced the future with NyquistAI, which was previously out of reach.
Please explore our pricing page. Discounts are available for enterprise users.
We offer flexible payment options, including credit card, debit card, bank transfer, and all payment methods accepted by Stripe. Contact [email protected] if you need additional payment support.
You can email [email protected] or use the Live Chat Support function in the platform for support. Available 9am-9pm ET M-F. Any after-hours messages receive replies within 24 hours.
Three times a day globally.
Yes. For entries beyond 300, please contact [email protected].
Founded on Innovation, Built with Passion
NyquistAI is a growing technology company providing an AI-powered solution to transform decades of clinical and regulatory data and documentation into actionable insights for Life Sciences professionals.